Connected Workplace for Life Sciences
Keep Your Operations Running Smoothly

Simplify Your Workplace
Better manage your facilities, labs, manufacturing plants, and quality processes by consolidating all space and asset information in one place.
Break Down Silos
Foster cross-team collaboration to reduce unscheduled manufacturing downtime. Gain full visibility into your workplace, and make the most of your spaces.
Optimize Operations
Connect assets, space, and data. Manage work orders, integrate calibration processes, automate workflows, and more.
Always Be Audit-Ready
Maintain accurate records and ensure data quality with built-in compliance controls, including Part 11 compliant audit trails and validation documentation.
The Only IWMS Built for Life Sciences
Create dynamic workspaces, streamline processes, ensure compliance, and make more informed decisions about your buildings and equipment, with one solution.
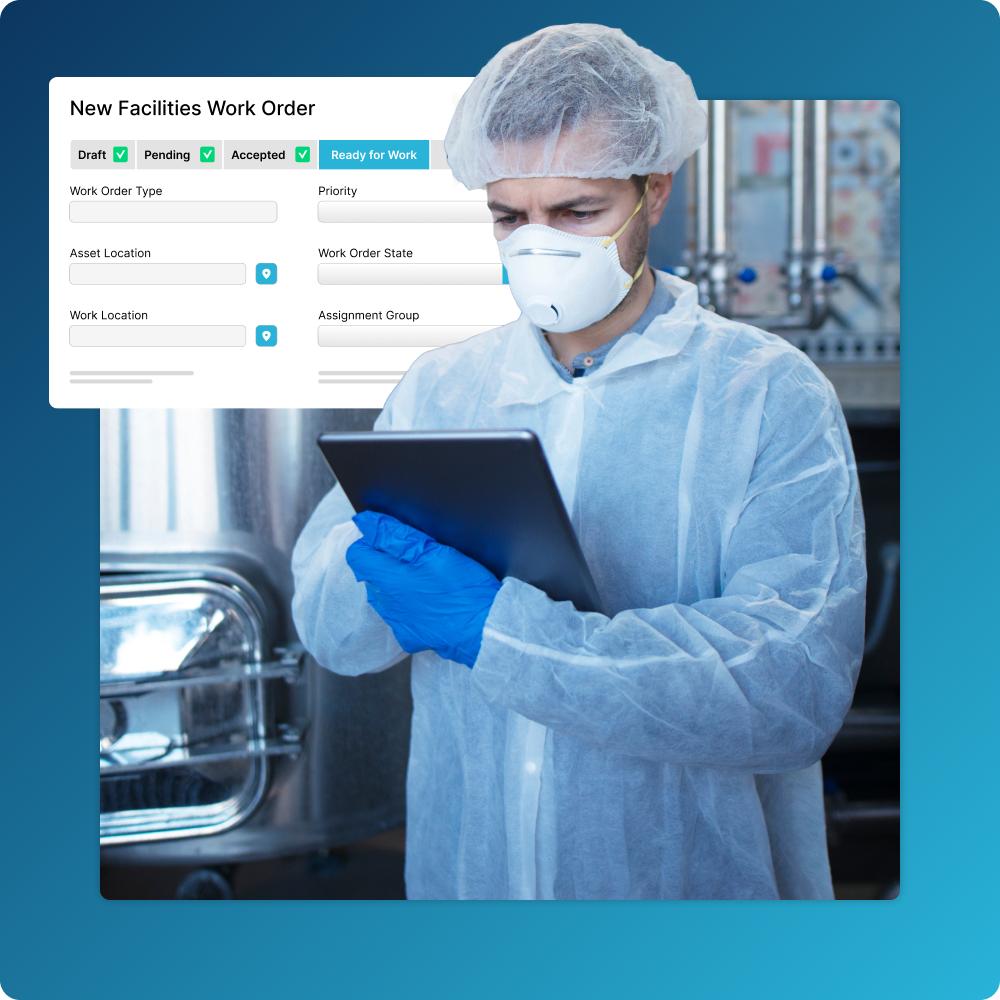
Streamline Your Maintenance Activities
Manage the full lifecycle of your GxP and non-GxP assets in one place.
- Easily indicate assets that have GxP applicability and create workflows that drive approvals, audit trails, and more.
- Find and access historical data about asset maintenance activity when you need it
- Facilitate compliance with electronic signatures that meet 21 CFR Part 11 and EU Annex 11 requirements
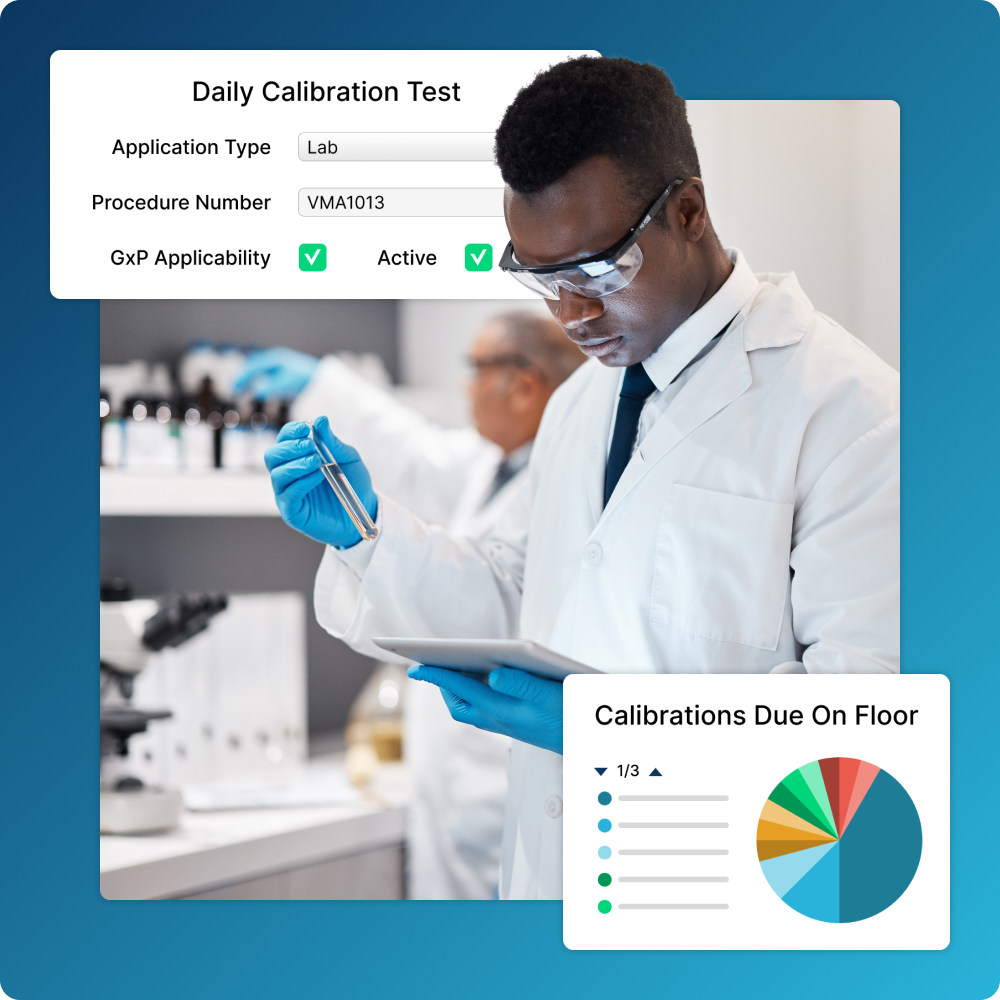
Integrate Calibration
Quickly get equipment back online by including calibrations in maintenance workflows.
- Centralize data so internal and external teams have access to the same information
- Improve data integrity and reduce redundant work with automatic calculations
- Generate calibration certificates to demonstrate compliance
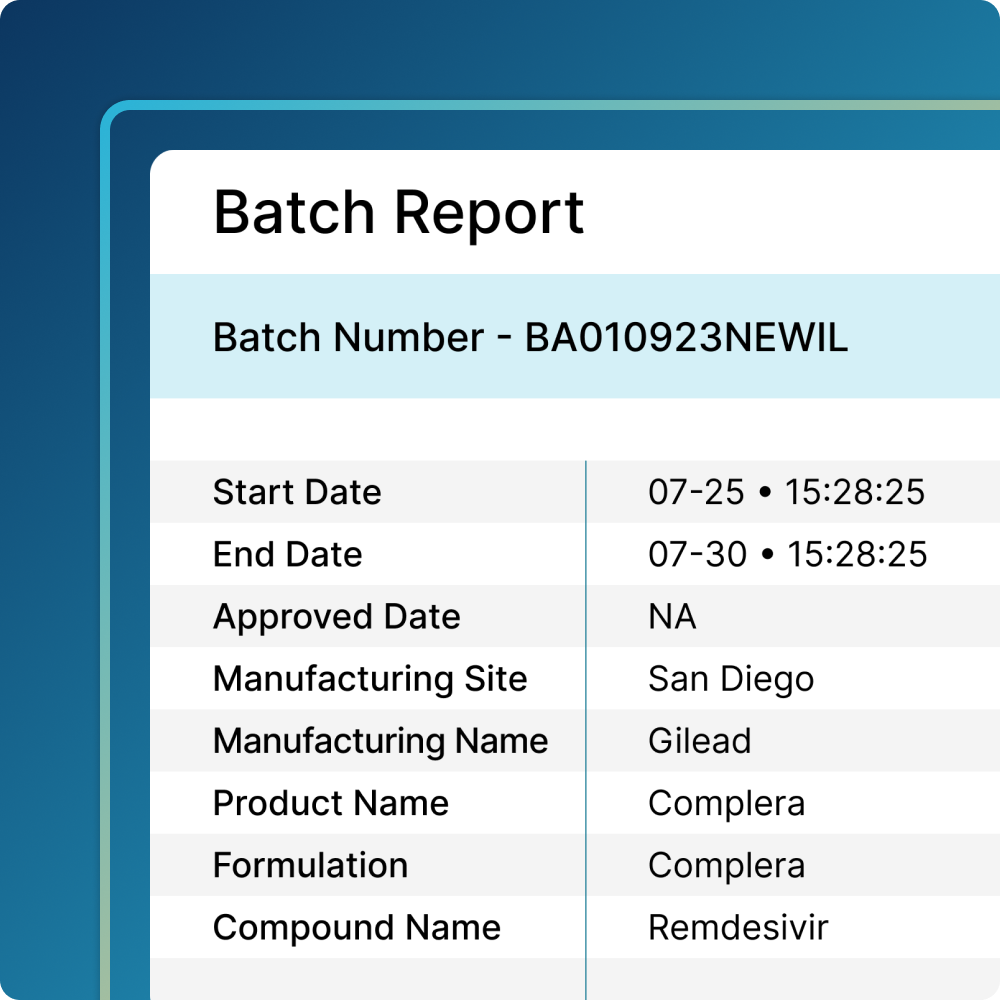
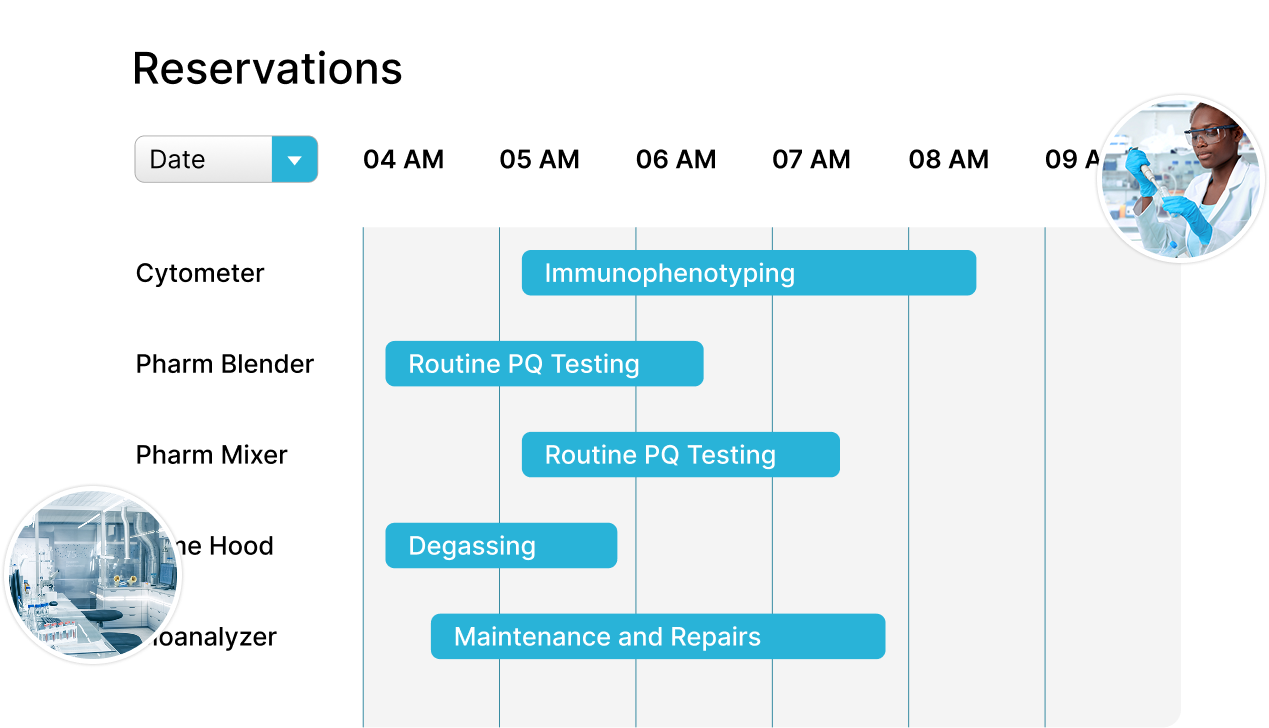
Align Quality and Manufacturing
Deliver products quickly, while maintaining the highest levels of quality.
- Simplify production by reserving the assets you need for every batch, every time
- Understand which assets are available for use based on risk factors determined by pre-defined rules
- Generate reports with assets used in each batch to support compliance and facilitate overall batch approval or rejection

Rethink Your Space Strategy
Make the most of your current spaces, plan for future workplace needs, and build a workplace with your employees in mind.
- Enable productivity by giving employees the ability to reserve the spaces they need to work
- Visualize, plan, and execute employee moves with intuitive tools and automatic workflows
- Capture key insights to help you rationalize and forecast how your spaces are used
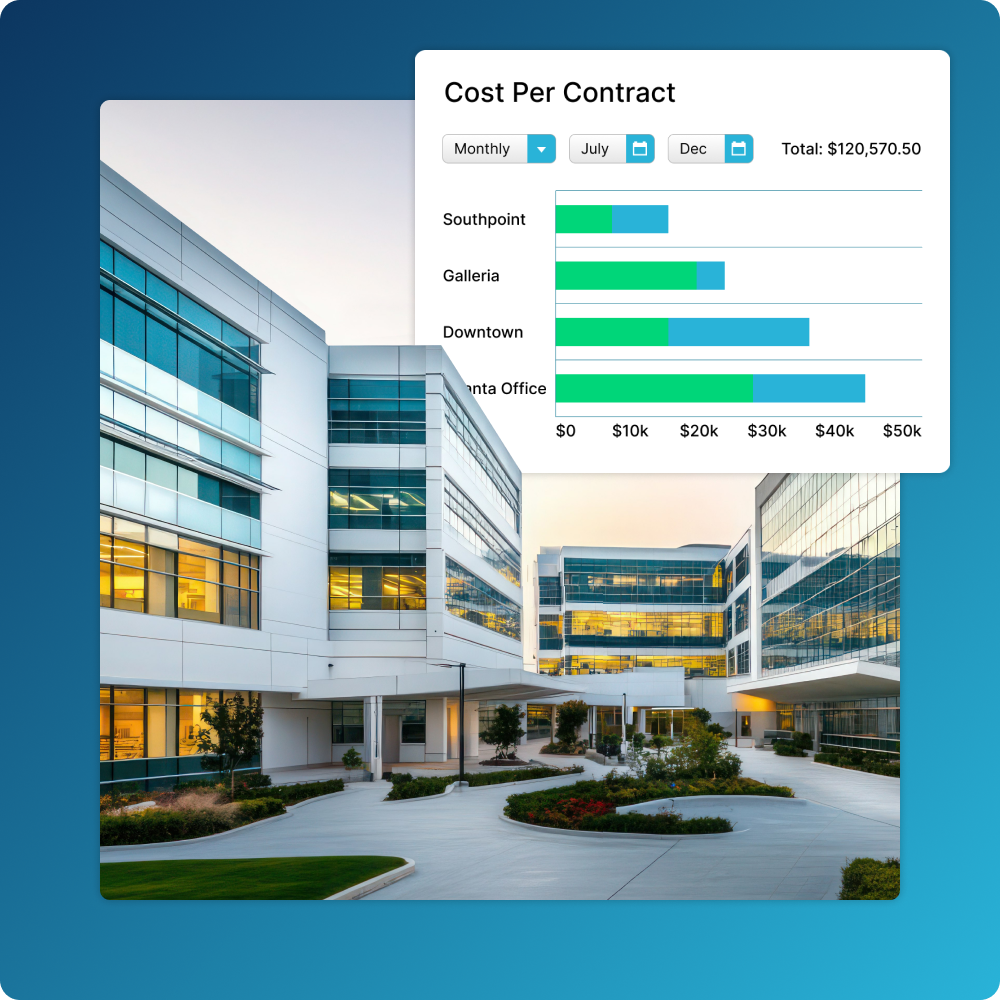
Know What’s in Your Lease Portfolio
Simplify management of retail lease lifecycles—including key details, payments, and critical dates—all while staying compliant with lease accounting standards.
- Create lease lifecycle workflows, set up custom lease scheduling and recurring payments, and get alerts for important dates and milestones
- Quickly classify contracts, execute individual payments, manage documents, and more from one centralized location
- Easily stay compliant with IFRS 16 and FASB lease accounting standards
See It In Action
Contact us for a demo to learn how Connected Workplace for Life Sciences can work for you.
REQUEST A DEMO