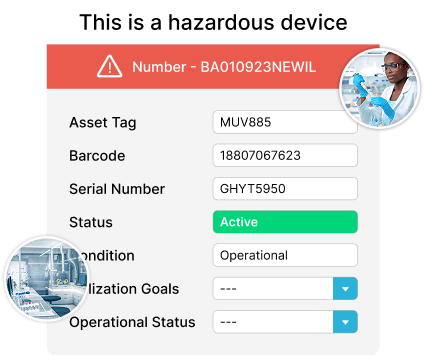
GxP Assessment Management
Maximize Asset Lifecycles
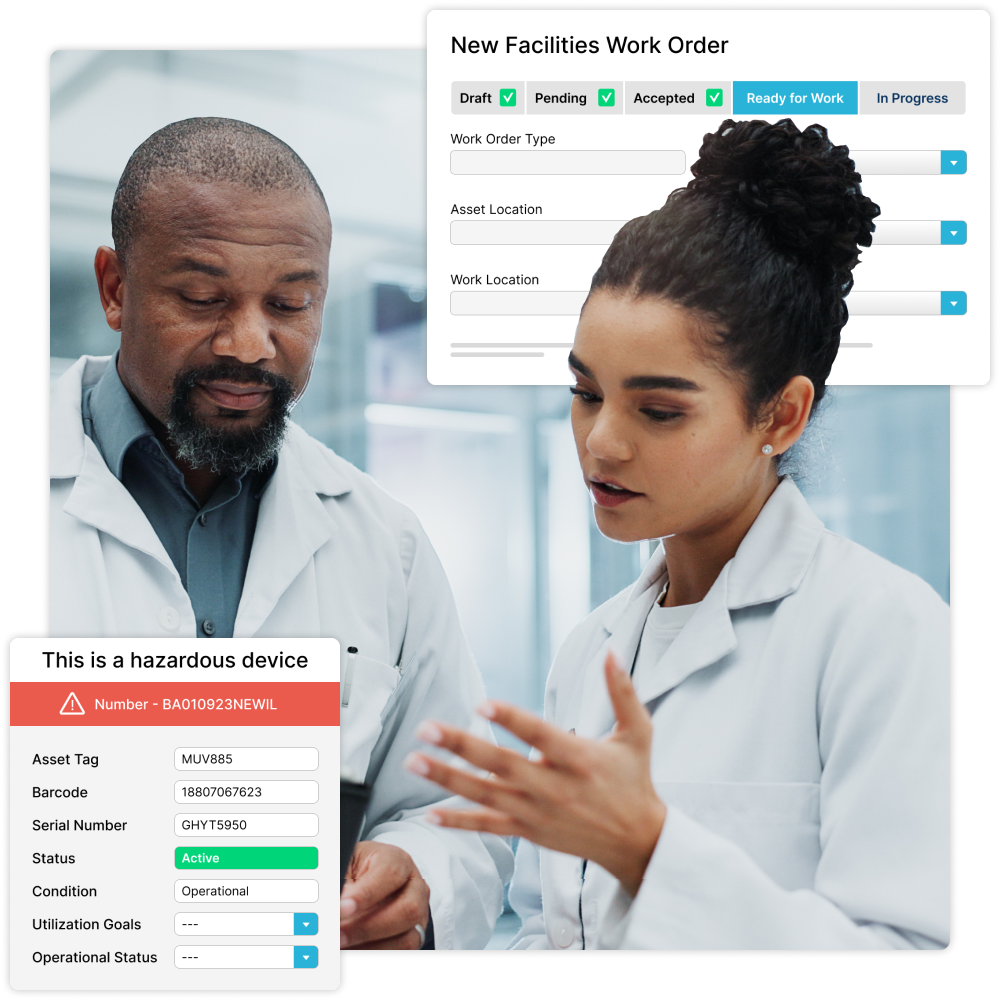
Say Goodbye to Separate Systems
Keep your facilities, labs, manufacturing plants, and quality processes running smoothly by consolidating all asset information in one place.
Break Down Silos
Reduce unscheduled downtime by coordinating across teams to ensure GxP and non-GxP equipment is available and reliable for production.
Increase Equipment Efficiency
Proactively schedule and auto-assign preventive maintenance, calibrations, and cleanings on your regulated and non-regulated equipment.
Always Be Audit-Ready
Maintain accurate records and ensure data quality with built-in compliance controls, including exportable audit trails and versioning.
Asset Management, Simplified
Create dynamic workspaces, streamline processes, ensure compliance, and make more informed decisions about your assets by consolidating your information.
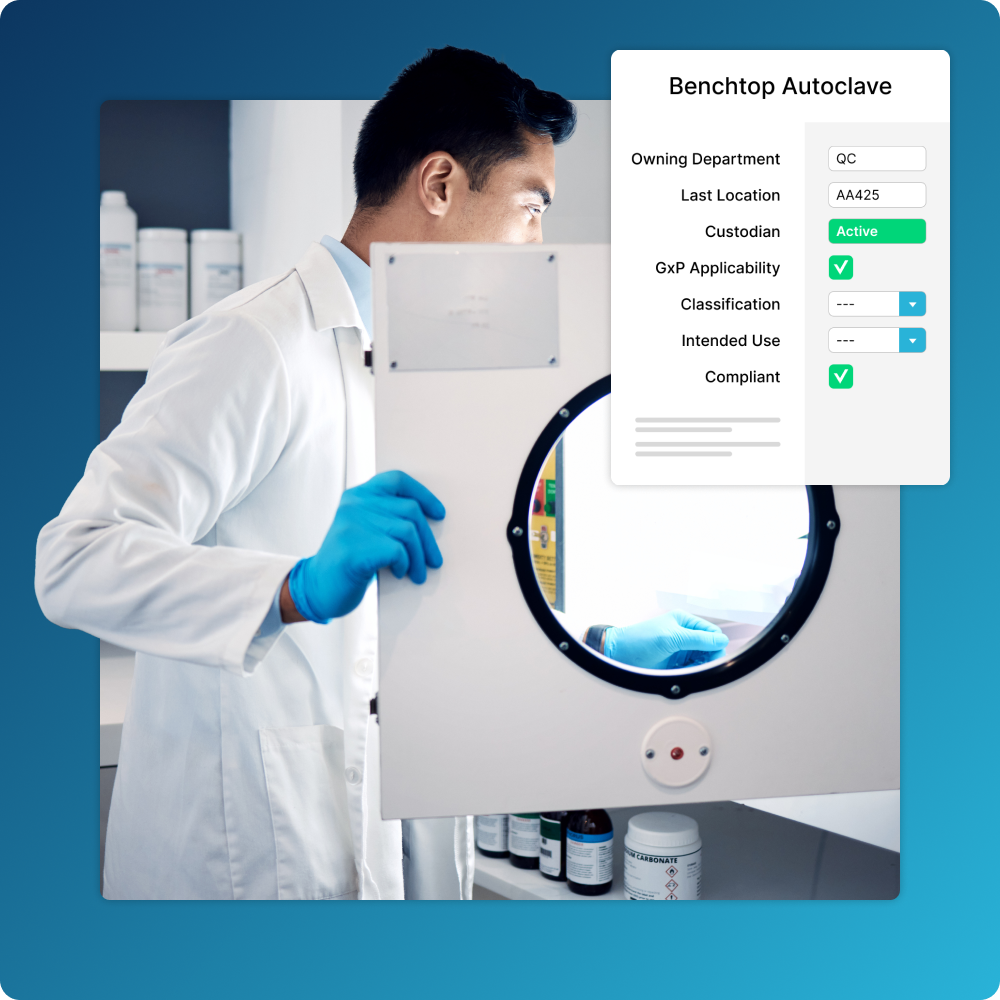
Compliance with a Click
Easily indicate assets that have GxP applicability and create workflows that drive approvals, audit trails, and more.
- Improve production and process controls, documentation, and work order management
- Find and access historical data about asset maintenance activity
- Facilitate compliance with electronic signatures that meet 21 CFR Part 11 and EU Annex 11 requirements
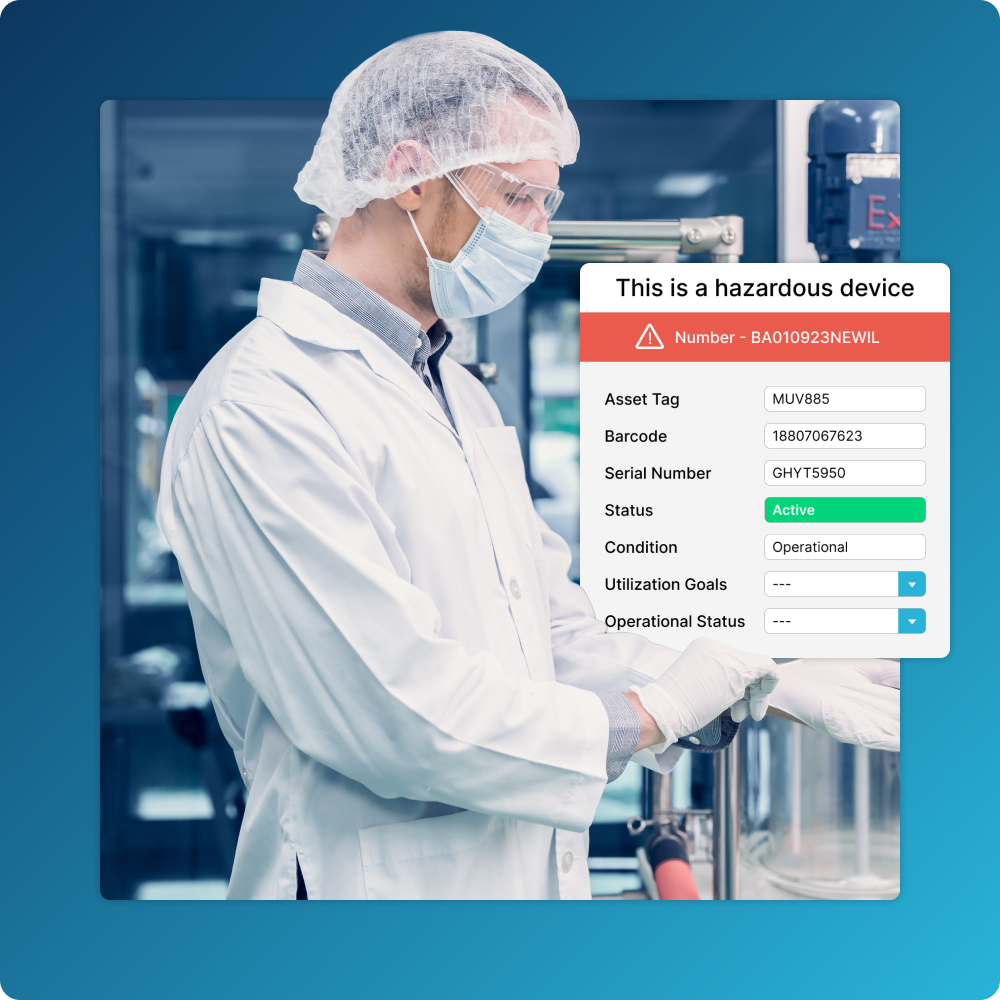
Centralized Asset Information
Track asset information such as costs, condition assessments, serial numbers, related service contracts, and more.
- Track and manage the condition of assets with pre-determined checklists
- Calculate and score risks associated with each asset based on its status
- Use data to understand performance trends and better predict potential issues
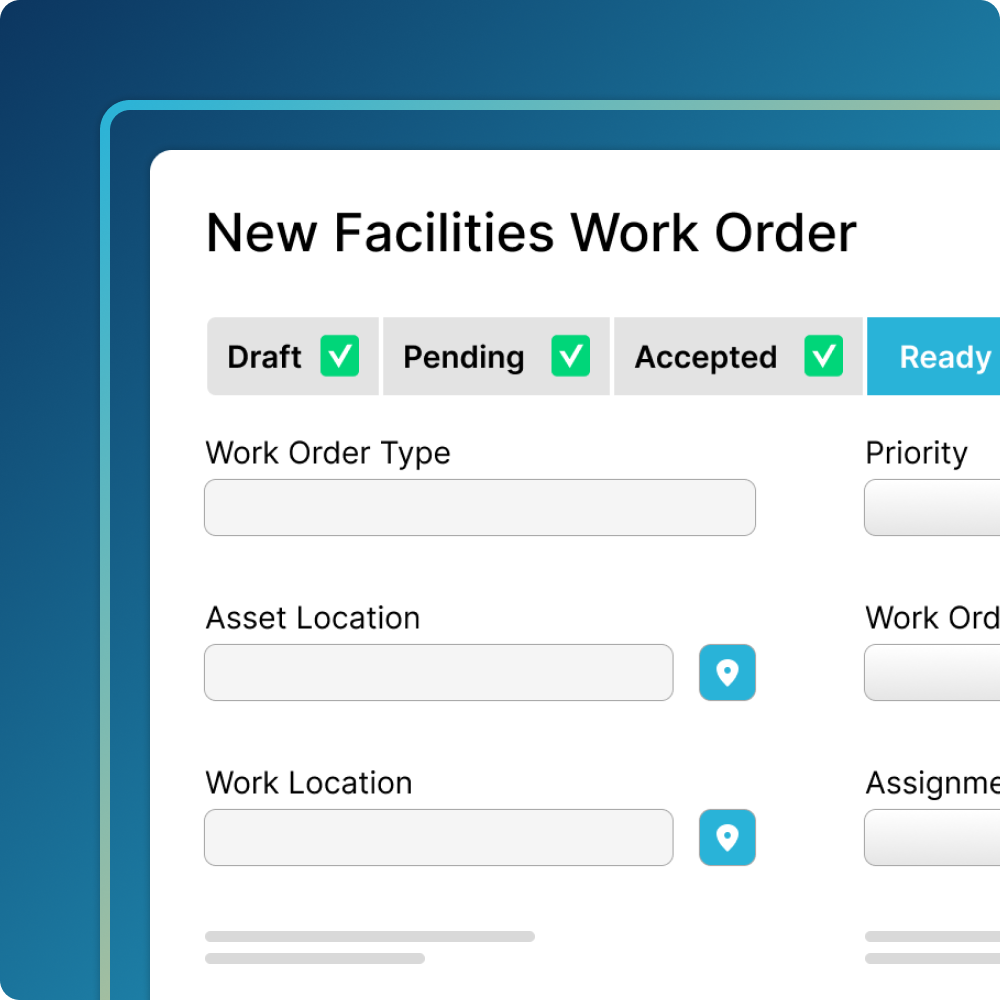
Comprehensive Maintenance
Plan and schedule maintenance while meeting regulatory requirements.
- Collaborate across teams to quickly remediate unexpected equipment issues
- Schedule maintenance, calibration, cleaning, and inspections at regular intervals to ensure that assets are operating correctly
- Maintenance data is recorded digitally, eliminating manual administrative work

Integrated Calibration
Embed calibration activities within maintenance workflows, so teams work with the same information.
- Bring equipment back online quickly
- Generate calibration certificates to demonstrate compliance
- Analyze failure trends to inform repair vs. replace decisions
See It In Action
Contact us for a demo to see how you can modernize your asset management strategy.
REQUEST A DEMO